|
|
Órgão Oficial de Divulgação Científica da
|
ISSN: 1679-1796
|
Laparoscopic Adaptive Gastro-omentectomy as
an Early Procedure to Treat and Prevent the Progress of Obesity
- Evolutionary and Physiological Support
Gastro-omentectomia Laparoscópica Adaptativa:
Novo Procedimento para Tratamento e Prevenção do Avanço da Obesidade
- Bases Fisiológicas e Evolucionárias
Sérgio Santoro1,2, Manoel C. P. Velhote1,3, Alexandre S. G. Mechenas2, Carlos E Malzoni1, Victor Strassmann1
Hospital Israelita Albert Einstein, Hospital da Polícia Militar, Hospital das Clínicas de São Paulo, São Paulo, Brasil
ABSTRACT
OBJECTIVE: This is the preliminary report of a new
surgical technique to treat and prevent the progress of
obesity: Laparoscopic Gastro-Omentectomy. The first case,
the evolutionary and physiological support to this technique
are shown. METHOD: The technique includes a
hand-assisted laparoscopic vertical (sleeve) gastrectomy and
omentectomy. RESULTS: A patient with BMI of 36
Kg/m2 was submitted to the procedure, discharged 48 hours after, with no complaints.
To date this patient lost 13 kg in 30 days following the
procedure and reports early satiety. CONCLUSIONS:
This is the preliminary report of a technique designed to abort the evolution of
obesity. The procedure does not create sub occlusions; no
prostheses are used and there are no excluded segments.
Neither malabsorption nor blind endoscopic areas are generated
and, fundamentally, no harm is done to important digestive
functions. Instead, it causes a modest gastric restriction that promotes
early satiety by distension and the procedure removes sources
of three important agents involved with obesity and its
comorbidities: Ghrelin, Plasminogen Activator Inhibitor-1 (PAI-1) and
Resistin. The operated patient will not need nutritional support or to
take pills chronically. The procedure is simple, fast and safe to perform.
Key words: OBESITY/surgery; MORBID OBESITY;
OMENTUM/surgery; GASTRECTOMY; GASTROPLASTY; ghrelin, human;
resistin; PAI-1; ADIPOSE TISSUE.
SANTORO S, VELHOTE MCP, MECHENAS ASG, MALZONI CE, STRASSMANN V. Laparoscopic Adaptive Gastro-omentectomy as an Early Procedure to Treat and Prevent the Progress of Obesity. Evolutionary and Physiological Support. Rev bras videocir 2003;1(3):96-102.
recent change in human diet increased
the participation of high caloric dense
food that is also easy to digest
and absorb. In the last thirty years food
abundance became common and it has led to a huge
increase in the incidence of obesity,
hypertension, diabetes, hypertriglyceridemia,
hypercholestero-lemia, and other conditions associated
with obesity. Nutritional Education and Medical Treatments are failing in avoiding obesity,
which led to the appearance of many techniques for surgical treatment of extreme obesity.
However, none of these techniques is easy, safe,
or physiologically correct enough to be applied
early in the development of obesity.
Extreme obesity is a personal tragedy. When it is present, the patient already
has damaged arteries, heart, joints, skin, etc.
Also, substantial psychological damage occurs
during the long process of weight gain. Current
surgical treatment is so aggressive that it can only
be offered when extreme obesity is present. Malabsorption, obstacles to food
ingestion, prosthesis to cause sub occlusions, and
excluded segments are troublesome. If we had a physiological, simple, easy and safe method
that could help stop the weight gain and start
some weight loss, without damage to digestive functions, we could possibly apply it sooner.
We could then, in effect, act soon and interfere
less. The procedure we are going to present here, in
a preliminary report, is the first step of a new strategy to face obesity, based on a new
concept called "Evolutionary Surgery". This
strategy, ongoing since October 2002, creates
different procedures for different stages and types
of obesity. This first step we are going to describe
is very simple and is based in physiological and evolutionary data.
Physiological Background
Ghrelin: This is a 28-amino peptide, predominantly produced by the stomach,
which displays strong growth hormone
(GH)-releasing activity, but also stimulates gastric acid
secretion, and is able to induce adiposity by activating
a central mechanism for increasing food intake and decreasing fat utilization
1, 2, 3. After a meal, ghrelin production falls. As time passes after
the last meal, its production is enhanced and it
has been shown that this participates in the
genesis of hunger 4. High level of ghrelin is not a
common cause for obesity since it was shown that
obese people have low levels of this hormone,
however, when significant weight loss occurs, ghrelin
levels go high, generating hunger and this is,
probably, a motive for weight regain 5.
PAI-1: Plasminogen activator inhibitor 1 (PAI-1) is the primary physiological inhibitor
of plasminogen activation, which means it is a pro-coagulant factor. Circulating PAI-1 levels
are elevated in patients with coronary heart
disease and it plays an important role in development
of atherothrombosis by decreasing fibrin
degradation 6. PAI-1 is produced mainly by visceral fat
tissue, mainly the omentum and mesenteric fat
6, 7, 8. Procedures that cause reduction in PAI-1
levels have already been pointed to improve
metabolic profile and reduce the cardiovascular risk
9, 10.
Resistin: It is clear today that adipose tissue is an endocrine gland and it produces
many substances that can act like hormones, such
as leptin, interleukin-6, adiponectin (also called ACRP30 and adipoQ), angiotensin II and
resistin. Resistin acts on skeletal muscle
myocytes, hepatocytes, and adipocytes themselves,
reducing their sensitivity to insulin and it is linked
to diabetes 11, 12. Abdominal fat is a main source
of resistin12, 13.
Visceral obesity: The fat tissue in the abdomen was clearly linked with what was
called Plurimetabolic syndrome. The waist to hip relation has been used to quantify
cardiovascular risk and many epidemiological studies
have pointed its relation to high blood pressure,
hyper-triglyceridemia, insulin resistance and
athero-thrombotic disease. Visceral fat is insulin
resistant and so, it keeps releasing free fatty acids
(FFA) to the portal system. It is believed that
insulin resistance of the liver derives from a
relative increase in the delivery of FFA from the
omental fat depot to the liver (via the portal
vein)14. Many extreme obese patients have quite good
metabolic profile because they have mostly
subcutaneous fat, while it is also clear that, except by
the orthopedic, respiratory and reflux
complications, most metabolic complications of obesity
are related to visceral fat.
Evolutionary Background
Primitive diet was raw, full of poorly digestible fiber, very hypocaloric and
highly contaminated. Big volumes of primitive diet
may have been isocaloric when compared to very
small volumes of modern diet. Stomachs were developed over time and they were prepared
to deal with primitive food. Our ancestors did not live in abundance and when facing good
food, the eating instinct was designed to eat
enough also to create a stockpile for long periods
of starvation. So, ingested volumes had to be massive and stomachs had to be big to fit
and process more food. Intense acid production was necessary to diminish the bacterial content
of food. In the stomach osmolarity of ingested
food is corrected and gastric emptying occurs
gradually and in a regulated manner, due to a
neuro-endocrine controlled pylorus.
Then, in a few centuries the human diet was deeply changed. Fire control made
food more digestible. Agriculture gave us some abundance and enhanced the participation
of carbohydrates. Refined sugar gave us in volume what nature could only give us in minimal
portions. Saturated fat and industrialized food worsened
the picture. Development of electric energy allowed
us to also eat at night. Marketing,
restaurants, cookies, and the other "goodies" of
Civilization represented a too fast change that our
Digestive system and our eating instincts could not follow.
Modern diet is hypercaloric, poor in fiber, easy to absorb and very little contaminated. In
the current environment, it seems that the gastric chamber is indeed too big and acid
production perhaps excessive.
Evolutionary Surgery - First step
We have been looking for ways we could adapt the digestive system and the
overeating instincts without causing damage to
precious digestive functions, like gastric, pyloric,
duodenal, ileal and colonic. Duodenum and proximal gut
have different functions than distal gut. Some
current techniques to treat obesity resect or exclude
pylorus (Scopinaro´s Bilio-pancreatic bypass, Fobbi
or Capella Roux-in-Y Gastric Bypass).
Biliopancreatic bypass, in the Scopinaro or Duodenal Swicth
mode, excludes the whole proximal gut and causes unspecific malabsorption that leads to
nutritional deficiencies. Gastric Bypass excludes most of
the stomach. Other techniques do not exclude, but
just provoke sub occlusion, like Gastric Banding.
The aggressiveness of these procedures impedes
them from being used in the early progress of obesity.
In our point of view, the many digestive functions are precious even to the obese.
However we admit that gastric capacity is bigger
than necessary when modern diet is used. Even the intestines, probably, are too long for
the modern diet15. Then, for early obesity, we propose a
vertical gastric resection - in the same mode
already extensively used in the "Duodenal
Switch" technique 16, associated to omentectomy.
METHODS
Technique
A six centimeters incision is made 2 cm under the left costal margin, parallel to it,
opening the abdominal cavity. A device developed to
allow the introduction of the hand in the abdomen without losing the pneumoperitoneum
(Lap Disk® - Ethicon Endo-Surgery, Inc.) is
inserted. A trocar sleeve is put in the Lap
Disk® and Lap
Disk® is closed, allowing inputting gas
and creating the 15 mm Hg pressure pneumo-peritoneum, in a safe way. Under direct
vision now, four other trocars are inserted (5mm in
the epigastric region - for liver retraction; 12 mm
in the right hypochondriac region for surgeon's
left hand and for stapling; 5 mm in the left hypochondriac region, lateral to the Lap
Disk® for an assistant; 5mm in the supra
umbilical position to the 5mm camera). The gastric
body and fundus are released from the greater
omentum and short gastric vessels by section of with
a harmonic scalpel (Ultracision® - Ethicon
Endo-Surgery, Inc.). After, the gastroepiploic arcade
is interrupted 6 cm proximal to the pylorus. Gastroepiploic vessels will remain intact in
the antrum. A Fouchet tube is passed through the esophagus until the duodenum by the
lesser curvature, with the help of surgeon's right
hand through the Lap Disk®. A linear cutting
stapler is used in order to resect gastric fundus and
most of gastric body, leaving a gastric tube of 3 to 4
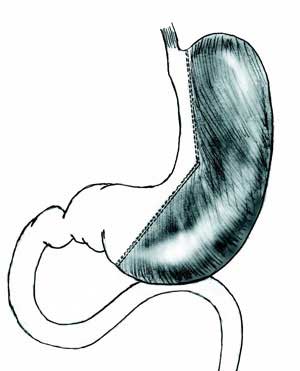
cm of diameter in the lesser curvature (Figure 1).
|
|
|
Figure 1: Scheme of gastric resection. |
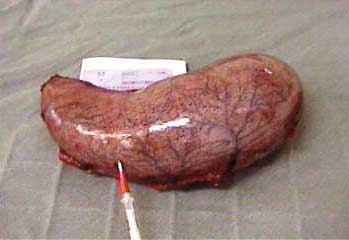
Gastric specimen is removed though the Lap
Disk® which is the taken away (Figure 2).
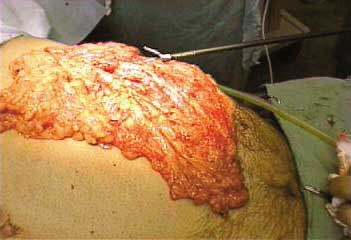
The great omentum is pulled out through the
incision and detached from the transverse colon
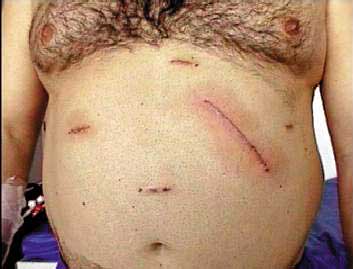
and removed as shown (Figure 3). Incisions are
closed with intradermic running suture and covered
with glue (Figure 4).
|
|
|
Figure 2: Stomach removed. |
|
|
|
Figure 3: Epiploon resection. |
|
|
|
Figure 4: Final aspect. |
The Ethical Committee of the "Hospital da Policia Militar do Estado de São Paulo"
approved the protocol. A detailed informed consent is
signed by patients, which states that weight loss cannot
be predicted because of lack of experience.
First Patient and Early Result
RMR, male, 34, Weight 111 Kg, Height 174 cm, BMI: 36.6
Kg/m2. He had been under many attempts of clinical treatment for ten years. By
the time of surgical indication he was taking
Orlistat. Sibutramine and others drugs had been
taken before. He could not lose any weight and he
began to present knee pain; no other comorbidity
was present but parents are diabetic and hypertensive.
Surgery took 120 minutes. He received cefazolin for 24 hours; he started taking liquids,
in small portions, in 24 hours and was discharged
in 48 hours.
He was oriented to take just liquids, not in more than 150ml at a time, for a week and then
he was allowed to eat solids. There was a recommendation to start meals with a portion
of salad. Fruits, vegetables, fish and chicken
were recommended. He refers early satiety and he
does not feel starving after more than 5 hours
without eating like he used to. In the first seven
days Omeprazole and metoclopramide were
prescribed. No medication was prescribed after that. After
30 days, he lost 13 Kg, his BMI is now 32.3
Kg/m2, and he is still losing weight. He is symptom free
and very much satisfied. Eating a lot less now had consequences on his metabolic profile.
Total cholesterol was 257 mg/dl and triglycerides, 212
mg/dl and 30 days after these results are
respectively 189 mg/dl and 115 mg/dl.
DISCUSSION
The procedure may bring many advantages and we believe it can adapt the stomach
to modern diet. Since food is now a lot more
caloric than primitive diet, gastric capacity is
reduced by more than 1 liter. However, there is no
sub occlusion, no stenosis and no prosthesis. The stomach is proportionally reduced, but it
keeps its general structure (cardia, body, antrum
and pylorus). Innervation by the lesser curvature
is intact. Satiety by gastric distension tends to occur with smaller volumes, because
these volumes, in modern diet, are enough. When significant weight loss occurs, lack of
elevation of Ghrelin production is expected because
its major source is removed 4, 5.
Omentectomy promotes resection of visceral fat, which is a source of PAI-1. It
is believed that less visceral fat and less PAI-1
are related to a reduced risk of atherothrombosis
6. Omentectomy also provides a reduction in
the source of Resistin and a source of free fat
acids to the portal vein. Both events are thought
to reduce hyperinsulinism and insulin resistance.
As the specimens removed are bulky, the intra abdominal pressure (IAP) is reduced. High
IAP is related to respiratory, hemodynamic and
reflux problems.
This first early result is very stimulating not just because patient is losing weight
without medication, surgical sub occlusions,
neither malabsorption, but also because a new concept
is involved. Surgeons, maybe for the first time,
are working not to treat a diseased organ, but to adapt a healthy one to a new environment.
The so-called "Evolutionary Surgery" protocol
is ongoing at the Hospital da Policia Militar do
Estado de São Paulo for nine months and other
obese patients have been operated using the other
steps in development. But just now, we operated on
a patient that, strictly, would have no indication to a "Bariatric Surgery" because he was not
sick enough (little comorbidties), nor fat enough
(BMI less than 40 Kg/m2) to be operated on.
However, this first step is simple, quite safe and it does
not harm any digestive function, but it indeed may help the patient to not get any fatter or even
to be more successful in his attempts to maintain an adequate weight.
We have to study the expected fall in ghrelin, resistin and PAI-1, as well as study
many patients for long periods, to know exactly
how helpful the procedure is. However, this
simple surgical procedure is helping the patient
to lose weight faster than any medication ever did.
It helps him eat less with early and prolonged
satiety, probably due to the removal of great deal of
the ghrelin releasing cells. Eating less will
probably impact cholesterol and triglycerides
levels enhancing the benefit of removing PAI-1 and Resistin producing cells. Nonetheless,
the improvement of metabolic profile will have to
be observed with a significant number of patients, as well as long-term results.
The diminishment of acid production is thought to be an evolution too. Modern diet
is almost sterile, when compared to the food in
the animal condition. As this food also is easily digested, the acid production of the modern
man is excessive, facing the kind of food man,
himself, developed in the last half century. Indeed,
excess of acid has been causing much more trouble
than the lack of it and this may be expressed by
the huge number of people taking acid reduction
pills, the incidence of peptic complaints and gastroesophageal reflux.
Gastroesophageal reflux may be prevented by this procedure. First, because it reduces
acid production. Second, because the tension in gastric fundus walls makes opposition to the
lower esophageal sphincter (LES) action. As this tension is proportional to the gastric
fundus diameter (Laplace´s Law) and this was very
much reduced, LES may get some extra efficacy.
Indeed, there are no reports of problems of reflux
after the Vertical (sleeve) Gastrectomy in the Duodenal Switch procedure. Nonetheless,
is important to say that there (differently than
here) they are protected against the alkaline reflux
by the duodenal exclusion. However, there are no special reasons to suppose that alkaline reflux
will be a problem here.
The current obesity surgical procedures are so aggressive that they cannot
be recommended to patients prior to extreme
obesity. So, by the time these procedures are
performed, the patient has already damaged arteries
and articulations, has already suffered the consequences of diabetes,
hypertension, dyslipidemias, reflux and other
obesity-associated conditions, besides the
psychosocial problems. With these medical problems,
the surgical risk is higher.
As the proposed procedure is simple and safe and as it maintains the general structure
and important digestive functions unharmed, with
no need of nutritional support, it can be recommended to patients before extreme
obesity, which reduces even more the surgical risk (already low by the procedure itself).
Extreme obesity is a severe condition, with some irreversible physical and psychological
damage developed over years. Avoiding its
development may be better than to treat it.
This may be a physiologically acceptable procedure not to treat extreme obesity but
to prevent it, before its development is obvious
or its associated diseases are evident.
In fact, this is an evolutionary and adaptive surgery that would not be
necessary had we had kept our primitive diet. It
might become a very important procedure since it
can help stop the development of obesity, hypertriglyceridemia, hypercholesterolemia,
type II diabetes, hypertension, atherothrombotic disease and other typical conditions of the
XX and XXI Centuries.
CONCLUSION
This is the preliminary report of a procedure that is the association of two well-known
procedures: Vertical Gastrectomy and Omentectomy. Both
are very simple and safe. Together, they produce a proportionately reduced stomach, however
without changing its general structure like other
obesity surgeries do. No stenosis, no sub occlusions,
no prosthesis, no excluded segments, no
malabsorption, no blind endoscopic areas and, fundamentally,
no harm to important digestive functions. The operated patient will not need nutritional
support or chronically take pills because of the
procedure. Removing the source of ghrelin and early
satiety through distension may help weight loss.
Weight loss and the removal of main sources of resistin
and PAI-1 may contribute to a better metabolic profile.
The "evolutionary procedure" intends
to adapt the stomach to modern diet and as it is
simple and safe it may be used early in the treatment
of obesity, possibly generating benefits in the
treatment of diabetes and in the atherothrombosis risk.
Acknowledgements: We thank Ethicon Endo-Surgery, Inc. for the donation of disposable
surgical equipment used and Mrs Muriel Hallet for correcting the English Version.
RESUMO
OBJETIVO: Esta é a comunicação preliminar de uma nova
técnica cirúrgica para tratar e prevenir o avanço da obesidade:
Gastro-Omentectomia Laparoscópica. O primeiro caso é relatado
assim como as bases fisiológicas e evolucionárias para o
procedimento. MÉTODO: A técnica inclui uma gastrectomia vertical
por grampeamento e a omentectomia realizada por laparoscopia
e auxiliada por equipamento que permite e uso auxiliar da mão
sem a perda do pneumoperitônio. RESULTADOS:
Um paciente com IMC 36 Kg/m2 foi operado, teve alta em 48 horas sem
queixas, perdeu, até agora, 13 quilogramas em 30 dias e refere
saciedade mais precocemente. CONCLUSÕES:
Este procedimento evita criar sub-estenoses, colocar próteses, excluir
segmentos digestivos do trânsito de nutrientes, gerar malabsorção
e fundamentalmente, evita prejudicar funções digestivas.
O procedimento gera uma restrição moderada, o que colabora
para a saciedade precoce por distensão gástrica com menores
volumes e visa também modificar as circunstâncias
neuroendócrinas, retirando grande parte da fonte produção de grelina, do
Inibidor da ativação do plasminogênio-1 (PAI-1) e da resistina. O
paciente operado não necessita suporte nutricional nem o uso crônico
de medicações. O procedimento é simples, seguro, rápido, além
de ser fácil de ser realizado.
Palavras-chave: OBESIDADE/cirurgia; OBESiDADE
MÓRBIDA; OMENTO/cirurgia; GASTRECTOMIA/cirurgia;
GASTROPLASTIA; grelina, humana; resistina; PAI-1; TECIDO ADIPOSO.
Bibliographic References
1. Muccioli G, Tschop M, Papotti M, Deghenghi R, Heiman
M, Ghigo E. Neuroendocrine and peripheral activities of
ghrelin: implications in metabolism and obesity. Eur J Pharmacol
2002; 440(2-3):235-54.
2. Penalva A, Baldelli R, Camina JP, et al. Physiology and
possible pathology of growth hormone secretagogues. J Pediatr
Endocrinol Metab 2001;14 Suppl 5:1207-12; discussion
1261-2.
3. Pinkney J, Williams G. Ghrelin gets hungry.
Lancet 2002;359(9315):1360-1.
4. Lustig RH. The neuroendocrinology of obesity. Endocrinol
Metab Clin North Am. 2001;30(3):765-85.
5. Cummings DE, Weigle DS, Frayo RS et al. Plasma ghrelin
levels after diet-induced weight loss or gastric bypass surgery. N
Engl J Med 2002;346(21):1623-30.
6. Juhan-Vague I, Alessi MC, Morange PE. Hypofibrinolysis
and increased PAI-1 are linked to atherothrombosis via
insulin resistance and obesity.Ann Med 2000;32 Suppl 1:78-84.
7. van Hinsbergh VW, Kooistra T, Scheffer MA, Hajo van Bockel
J, van Muijen GN. Characterization and fibrinolytic
properties of human omental tissue mesothelial cells. Comparison
with endothelial cells. Blood 1990;75(7):1490-7.
8. Juhan-Vague I, Alessi MC. PAI-1, obesity, insulin resistance
and risk of cardiovascular events. Thromb Haemost 1997;78(1):656-60.
9. Carmichael AR, Tate G, King RF, Sue-Ling HM, Johnston
D. Effects of the Magenstrasse and Mill operation for obesity
on plasma plasminogen activator inhibitor type 1,
tissue plasminogen activator, fibrinogen and insulin.
Pathophysiol Haemost Thromb 2002;32(1):40-3.
10. Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P.
A pilot study of long-term effects of a novel obesity
treatment: omentectomy in connection with adjustable gastric
banding. Int J Obes Relat Metab Disord 2002;26(2):193-9.
11. Shojima N, Sakoda H, Ogihara T et al. Humoral regulation
of resistin expression in 3T3-L1 and mouse adipose cells.
Diabetes 2002;51(6):1737-44.
12. McTernan PG, McTernan CL, Chetty R, et al. Increased
resistin gene and protein expression in human abdominal adipose
tissue. J Clin Endocrinol Metab 2002;87(5):2407.
13. McTernan CL, McTernan PG, Harte AL, Levick PL,
Barnett AH, Kumar S. Resistin, central obesity, and type 2
diabetes. Lancet 2002;359(9300):46-7.
14. Bergman RN, Van Citters GW, Mittelman SD, et al.
Central role of the adipocyte in the metabolic syndrome. J
Investig Med 2001;49(1):119-126.
15. Santoro, S. Relações entre o comprimento do intestino e
a obesidade. Hipótese: a Síndrome do Intestino Longo.
Einstein, 2003;1(1):58-9.
16. Marceau P; Hould FS; Potvin M; Lebel S; Biron
S. Biliopancreatic diversion (duodenal switch procedure). Eur
J Gastroenterol Hepatol, 1999; 11(2): 99-103.
ENDEREÇO PARA CORRESPONDÊNCIA
SÉRGIO SANTORO
Rua São Paulo Antigo, 500 apt. 111 SD
São Paulo, SP
Brazil
CEP 05.684-010
e-mail: ssantoro@ajato.com.br
(1) Hospital Israelita Albert Einstein, São Paulo, Brasil.
(2) Hospital da Polícia Militar do Estado de São Paulo, Brasil.
(3) Instituto da Criança, Hospital das Clínicas, São Paulo, Brasil.
Recebido em 15/09/2003
Aceito para publicação em 25/09/2003